|
Coronavirus COVID-19 - BREAKING NEWS!!!
|
Posts: 2,092
Threads: 526
Thanks Received: 7,125 in 2,067 posts
Thanks Given: 1,791
Joined: 14 August 18
WHO says pandemic getting worse globally, urges countries to continue efforts
Quote:The World Health Organization on Monday warned against complacency and urged countries to continue their efforts to curb the spread of the novel coronavirus as the pandemic was getting worse globally, Reuters reported.
There are more than 71.21 lakh coronavirus cases worldwide. Covid-19 has killed over 4.06 lakh people, according to the Johns Hopkins University tracker.
WHO Director General Tedros Adhanom Ghebreyesus said that more than 1,36,000 cases were reported worldwide on Sunday, and this was the most in a single day so far. “More than six months into the pandemic, this is not the time for any country to take its foot off the pedal,” he said in an online briefing.
More below,
https://scroll.in/latest/964206/coronavi...ue-efforts
Johns Hopkins University tracker
https://www.arcgis.com/apps/opsdashboard...62e5c06e61
COVID-19: WHO clarifies comments on asymptomatic spread
Quote:Studies show people with the coronavirus are most infectious just at the point when they first begin to feel unwell, World Health Organization (WHO) experts said on Tuesday (Jun 9).
This feature has made it so hard to control spread of the virus that causes COVID-19 disease, but it can be done through rigorous testing and social distancing, they said.
"It appears from very limited information we have right now that people have more virus in their body at or around the time that they develop symptoms, so very early on," Maria van Kerkhove, a WHO epidemiologist and technical lead on the pandemic, told a live session on social media.
Preliminary studies from Germany and the United States suggest that people with mild symptoms can be infectious for up to 8-9 days, and "it can be a lot longer for people who are more severely ill", she said.
More below,
https://www.channelnewsasia.com/news/wor...d-12819702
Posts: 2,092
Threads: 526
Thanks Received: 7,125 in 2,067 posts
Thanks Given: 1,791
Joined: 14 August 18
Coronavirus vaccine: a bit of information and status
CNN's Health Correspondent, Holly Yan shares us rlevant information on COVID-19 vaccine.
When will a Covid-19 vaccine be available to the public?
No one's sure yet, but the target is sometime in early 2021. Vaccines in development around the world are in various stages of testing. Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said he's confident one of the vaccine candidates will be proven safe and effective by the first quarter of 2021.
Why does it take so long to develop a vaccine?
Vaccines have to go through multi-phase trials to make sure they're effective and safe. Typically, a vaccine takes eight to 10 years to develop, said Dr. Emily Erbelding, an infectious disease expert at the NIAID.
Here's how the process typically works:
First, a vaccine is usually tested in animals before humans. If the results are promising, a three-phase trial in humans will begin:
Phase 1: The vaccine is given to a small group of people to assess safety and, sometimes, immune system response. If things go well, researchers move on to:
Phase 2: This phase increases the number of participants -- often into the hundreds -- for a randomized trial. More members of at-risk groups are included. "In Phase II, the clinical study is expanded and vaccine is given to people who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended," according to the Centers for Disease Control and Prevention.
What are the dangers of rushing the process?
History has shown that vaccines developed or distributed in a hurry can lead to unintended consequences:
-- In 2017, a rushed campaign to vaccinate about 1 million children for mosquito-borne dengue in the Philippines was stopped for safety reasons. The Philippine government indicted 14 state officials in connection with the deaths of 10 vaccinated children, saying the program was launched "in haste."
-- In 1976, the US was dealing with a novel swine flu outbreak. President Gerald Ford's administration ignored a warning from the World Health Organization and vowed to vaccinate "every man, woman and child in the United States" against the new virus. After 45 million people were vaccinated, researchers discovered a disproportionately high number of them -- about 450 people -- had developed Guillain-Barré syndrome
So how do we safely speed up the process?
"No vaccine is going to be put forward unless it's been checked out very thoroughly, both in terms of 'Is it safe?' and 'Does it protect you?'" said Collins, the director of the National Institutes of Health.
Scientists are trying to find safe ways of speeding up the typical processes.
Who's making the vaccines?
Dozens of research teams from around the world are working to develop or test coronavirus vaccines. As of early June, there were more than 120 candidate vaccines. "Because we have a number of these (trials), and they all use a different strategy, I am optimistic that at least one, maybe two, maybe three will come through looking like what we need," Collins said.
More below,
https://edition.cnn.com/2020/06/08/healt...index.html
Posts: 81
Threads: 10
Thanks Received: 49 in 45 posts
Thanks Given: 38
Joined: 18 October 18
03 February 21, 10:13
(This post was last modified: 08 February 21, 17:13 by jasonX.)
Quote:
Philippines' coronavirus cases top 527,000
The Philippines now has 527,272 confirmed COVID-19 cases after the Department of Health (DOH) reported 1,658 new cases on Monday, February 1.
The DOH reported 58 new deaths due to the coronavirus disease, bringing the death toll to 10,807, while the recoveries are up by 27, raising total recoveries to 487,574.
Of the total cases, 28,891 are active. "
Source
![[-]](https://www.geeks.fyi/images/collapse.png) The following 1 user says Thank You to mjcn19 for this post:1 user says Thank You to mjcn19 for this post
• harlan4096
The following 1 user says Thank You to mjcn19 for this post:1 user says Thank You to mjcn19 for this post
• harlan4096
Posts: 2,092
Threads: 526
Thanks Received: 7,125 in 2,067 posts
Thanks Given: 1,791
Joined: 14 August 18
(03 February 21, 10:13)mjcn19 Wrote: Quote:Philippines' coronavirus cases top 527,000
The Philippines now has 527,272 confirmed COVID-19 cases after the Department of Health (DOH) reported 1,658 new cases on Monday, February 1.
The DOH reported 58 new deaths due to the coronavirus disease, bringing the death toll to 10,807, while the recoveries are up by 27, raising total recoveries to 487,574.
Of the total cases, 28,891 are active. "
Source
I checked your "Source",
![[Image: 2021-02-09-004548.png]](https://i.postimg.cc/MGK8Q4XN/2021-02-09-004548.png) TIP: Try to post a world trend (not just your country).
TIP: Try to post a world trend (not just your country).
Posts: 2,092
Threads: 526
Thanks Received: 7,125 in 2,067 posts
Thanks Given: 1,791
Joined: 14 August 18
08 February 21, 22:05
(This post was last modified: 08 February 21, 22:12 by jasonX.)
COVID-19 Vaccines: Some information from around the net
COVID-19 is scary. Thank God that vaccinations have been rolling out. In other countries it is still months (or quarters) away
Governments around the world are now going "ga-ga" about purchasing and starting vaccination programs for their respective countrymen. Nations that are well-off or termed "first world" like the G8(G9) countries never have to worry about it as they have the money and capability (of course "influence") to get what they want and do as they please for their respective countrymen. A lot of competition also for the drug manufacturers as they "boast" of effectivity even with "unfinished" testing data. And recently there has been reported deaths in Norway for old people who were jabbed with Pfizer's vaccine. More confusion...more fear (in addition to COVID-19 and the mutated variants!).
Some FAQs are below:
Quote:- Can a COVID-19 vaccine make me sick with COVID-19?
No. None of the authorized and recommended COVID-19 vaccines or COVID-19 vaccines currently in development in the United States contain the live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19.
There are several different types of vaccines in development. All of them teach our immune systems how to recognize and fight the virus that causes COVID-19. Sometimes this process can cause symptoms, such as fever. These symptoms are normal and are a sign that the body is building protection against the virus that causes COVID-19. Learn more about how COVID-19 vaccines work.
- After getting a COVID-19 vaccine, will I test positive for COVID-19 on a viral test?
No. Neither the recently authorized and recommended vaccines nor the other COVID-19 vaccines currently in clinical trials in the United States can cause you to test positive on viral tests, which are used to see if you have a current infection.
- If I have already had COVID-19 and recovered, do I still need to get vaccinated with a COVID-19 vaccine?
Due to the severe health risks associated with COVID-19 and the fact that re-infection with COVID-19 is possible, vaccine should be offered to you regardless of whether you already had COVID-19 infection. CDC is providing recommendations to federal, state, and local governments about who should be vaccinated first.
At this time, experts do not know how long someone is protected from getting sick again after recovering from COVID-19. The immunity someone gains from having an infection, called natural immunity, varies from person to person.
- Will a COVID-19 vaccination protect me from getting sick with COVID-19?
Yes. COVID-19 vaccination works by teaching your immune system how to recognize and fight the virus that causes COVID-19, and this protects you from getting sick with COVID-19.
- Will a COVID-19 vaccine alter my DNA?
No. COVID-19 mRNA vaccines do not change or interact with your DNA in any way.
Messenger RNA vaccines—also called mRNA vaccines—are the first COVID-19 vaccines authorized for use in the United States. mRNA vaccines teach our cells how to make a protein that triggers an immune response.
- Is it safe for me to get a COVID-19 vaccine if I would like to have a baby one day?
Yes. People who want to get pregnant in the future may receive the COVID-19 vaccine.
Based on current knowledge, experts believe that COVID-19 vaccines are unlikely to pose a risk to a person trying to become pregnant in the short or long term. Scientists study every vaccine carefully for side effects immediately and for years afterward. The COVID-19 vaccines are being studied carefully now and will continue to be studied for many years, similar to other vaccines.
Content source: Centers for Disease Control and Prevention
https://www.cdc.gov/coronavirus/2019-nco...facts.html
Authorized and Recommended Vaccines
The US CDC (Centers for Disease Control and Prevention) provides the info below as guide for us. Thus it will be important to understand what is known about each vaccine. In the US, the CDC will provide information on who is and is not recommended to receive each vaccine and what to expect after vaccination, as well as ingredients, safety, and effectiveness.
Currently, two vaccines are authorized and recommended to prevent COVID-19:
- Pfizer-BioNTech COVID-19 vaccine
- Moderna’s COVID-19 vaccine
Vaccines in Phase 3 Clinical Trials
As of December 28, 2020, large-scale (Phase 3) clinical trials are in progress or being planned for three COVID-19 vaccines in the United States:
- AstraZeneca’s COVID-19 vaccine
- Janssen’s COVID-19 vaccine
- Novavax’s COVID-19 vaccine
Source and more reading here:
Pfizer: Coronavirus Vaccine Proves Effective Against Virus Mutations
https://www.usnews.com/news/health-news/...-mutations
Comparing the Covid-19 vaccines developed by Pfizer, Moderna, and Johnson & Johnson
https://www.statnews.com/2021/02/02/comp...n-johnson/
What about Chinese vaccines?
Authorities of the FDA approved a vaccine developed by Sinopharm's Beijing affiliate as early as December 24th last year, a day after the developer said interim analysis of its Phase 3 trial showed 79.34% efficacy, without providing details. The efficacy rate is lower than the 86% rate for the same vaccine announced by the United Arab Emirates on December 9, based on preliminary data. A Sinopharm executive said detailed data would be released later without giving a specific timeline.
In January, Sinopharm said that the vaccine’s ‘protective efficacy’ against COVID-19 was at 79.34%, slightly less than the previously reported efficacy rate of 86% in UAE (December 9 data).
Sinovac's candidate has also showed varied efficacy readouts. Data from a late-stage trial of its CoronaVac shot in Turkey showed a 91.25% success rate, while researchers in Brazil said its efficacy was between 50% and 90%.
Sinovac vaccine showed an effectivity rate significantly less than previous data suggested - barely over the 50% needed for regulatory approval.
Reuters publication on China's Sinovac and Sinopharm vaccines
China’s COVID-19 vaccines based on the inactivated virus can be upgraded to cope with new variants in about two months, the Global Times reported on Tuesday citing an expert with the Chinese Center for Disease Control and Prevention.
There are concerns that vaccines developed over the last year may be less effective against new variants of the virus discovered recently in Britain and South Africa. Moderna Inc said previously it would test a new booster shot aimed at the South African variant after concluding the antibody response could be diminished.
Vaccines from Sinovac Biotech (Sinovac vaccine) and China National Pharmaceutical Group (Sinopharm), which are being used in China and overseas, contain the inactivated virus that cannot replicate in human cells.
Source and more reading here:
Reuters Article
https://www.reuters.com/article/us-healt...SKBN29V14L
A Second Chinese Coronavirus Vaccine Is Said to Be Effective
https://www.nytimes.com/2021/01/07/busin...novac.html
Sinopharm’s COVID-19 vaccine scores approval in China
http://www.pmlive.com/pharma_news/sinoph...%20Bahrain.
Sinovac: Brazil results show Chinese vaccine 50.4% effective
https://www.bbc.com/news/world-latin-america-55642648
Sputnik V vaccine
Before it was reported that the Russian vaccine "Sputnik V" has "92% efficacy rate" data during the trials, the Russian governement efforts has been widely criticized for being rushed, elevating nationalistic competition over scientific evidence. Russia's Sputnik V is being rolled out to other countries even without the complete data from the trials. Last February 3, British medical journal, the Lancet stated in their peer-reviewed journal that Sputnik V, was "92" percent effective at preventing symptomatic illness in a large clinical trial, robust protection that puts it in line with top vaccines developed in the United States and Europe.
Read info below.
Source and more reading here: Russia's Sputnik V vaccine has 92% efficacy in trial
https://www.bbc.com/news/health-55900622
Novavax vaccine
The Novavax vaccine Phase 3 trial results from the UK and Phase 2b results from South Africa were recently announced with varied results.
Quote:Novavax vaccine trials run in South Africa and the UK indicate that its efficacy in the UK was 89% at least seven days after individuals had received two doses of vaccine. In South Africa, the vaccine efficacy was 60% in people living without HIV. A small group of individuals living with HIV – about 150 – was included in the efficacy analysis. However, the study didn’t have the statistical power to evaluate for vaccine efficacy specifically in this population.
Why the major difference in risk between the UK’s 89% and South Africa’s 60%?
Read info below.
Source and more reading here: Results from Novavax vaccine trials in the UK and South Africa differ: why, and does it matter?
https://theconversation.com/results-from...ter-154293
Single does vaccine: How effective is a single dose of each of the Covid-19 vaccines?
One jab and you can go on with your life, correct? No. That is not that simple while the trend for drug manufacturers to "also" manufacture a single does vaccine in addition to their 2-does vaccines, clinical trials have yet to start and data isn't yet solid. In my opinion, a single does vaccine is a drug manufacturer's way of limiting the cost of producing, testing the vaccine they are developing...but what do I know? I'm just a news reader!
So do read some comparison's done by BBC below.
Quote:...At a time when the answer is more urgent than ever – especially as the British government has decided to delay the second dose of all currently approved Covid-19 vaccines from 3-4 weeks to 12, and Russia is trialling a single-dose regimen of its Sputnik V vaccine named "Sputnik-Light" – it's also surprisingly complicated. Here's what we know so far.
Pfizer-BioNTech
According to Pfizer data published in December 2020, the Pfizer-BioNTech vaccine is roughly 52% effective after the first dose. Out of 36,523 participants in the phase three trial – the final stage of testing where people either received two full doses, 21 days apart, or a placebo – who had no evidence of existing infection, 82 people in the placebo group and 39 in the vaccine group developed Covid-19 symptoms. However, this early protection comes with some important caveats. First, the protection doesn't kick in until at least day 12 – until then, there was no difference between the two groups. Secondly, one dose is still significantly less protective than two. The latter is 95% effective at preventing the disease after a week.
But there is also another figure that has been circulating on the internet, and anecdotally, being fed to patients by certain doctors – the suggestion that the first dose is around 90% effective. And this is where it gets a little more complicated.
Oxford-AstraZeneca
For the Oxford-AstraZeneca vaccine, things are a bit different. In a paper published in January, the authors explain that the vaccine offers protection of 64.1% after at least one standard dose. This compares to 70.4% if you've had two full doses, or – oddly – 90% in people who have had one half dose followed by one full dose.
Meanwhile, based on these unpublished data they have seen, the Vaccine Committee has estimated that, from three weeks until 9-12 weeks after the first injection, the vaccine prevents around 70% of cases of serious disease.
Because the phase three trial included two gaps between the first and the second dose – including one of six weeks and a longer one of 12 weeks – it's possible to say with more certainty that the first dose can continue to provide some protection for at least a few months before the booster shot.
Moderna
According to a document the company submitted to the FDA, the Moderna vaccine can provide 80.2% protection after one dose, compared to 95.6% after the second (in people aged 18 to 65 – it's 86.4% in those over 65). As with the Pfizer vaccine, all participants in the phase three trial received two doses of the vaccine or a placebo within a single set time period – in this case, 28 days – so it's not yet known whether the immunity from a single vaccine would continue, or drop off after this stage.
Sinovac
The CoronaVac vaccine was developed by Sinovac, a biopharmaceutical company based in Beijing, China. This version is unusual as it has been trialled independently in several countries – all of which have produced different results.
According to researchers in Turkey, the vaccine is 91.25% protective, while scientists in Indonesia have said that it’s 65.3% effective, and the Butantan Institute in São Paulo, Brazil recently announced that the vaccine prevents 50.4% of people from developing symptoms. At the moment, no one has released data on the efficacy of a single dose – these figures only apply to two doses, spaced 14 days apart.
The results have been viewed with some scepticism, because they were published via press releases, instead of – as would normally be the case – in a peer-reviewed journal. Without access to more information about the trial methods and the data that was collected, it’s harder for scientists to make their own assessments of the results' validity.
Sinopharm
In all, there are five Chinese vaccines at various stages of development.
Another is "BBIBP-CorV", by the state-owned company Sinopharm, based in Shanghai. Officials in the country recently announced that this version is 79% effective after two doses – though by then, it had already been distributed to nearly a million people. This estimate has not been verified by the international community, because the underlying data and methods for its trial have not been made publicly available. It's not yet clear how protective it might be after a single dose.
Outside China, the vaccine is currently being tested all over the world, and has been approved in Bahrain, Egypt, Jordan, the Seychelles, and the United Arab Emirates. The UAE recently became the first to rate its efficacy, claiming via a press release that it is 86% effective...
Source and more reading here: How effective is a single vaccine dose against Covid-19?
https://www.bbc.com/future/article/20210...ccine-dose
Source and more reading here: UPDATED Comparing COVID-19 Vaccines: Timelines, Types and Prices
https://www.biospace.com/article/compari...sputnik-v/
India's Covaxin or Covishield
Now in India there has been confusion due to the fact that the Indian government authorized two vaccines on Jan 3: Covishield, the AstraZeneca-Oxford University vaccine manufactured by Pune-based Serum Institute of India, and Covaxin, an indigenous vaccine candidate developed by Hyderabad-based Bharat Biotech International.
Covaxin has been approved for restricted emergency use, although its efficacy trial is not complete. It is being rolled out "on clinical trial mode". Those getting Covaxin have to sign a form consenting to be treated as trial volunteers. If they refuse, they get no Covid-19 vaccine at all.
Quote:Covishield
This vaccine has been developed by the University of Oxford and British-Swedish pharmaceutical company AstraZeneca. Adar Poonawalla, Chief Executive Officer, Serum Institute of India (SII) -- which is British-Swedish pharma giant AstraZeneca's manufacturing partner – said that the vaccine would be 90 to 95 per cent effective if the two shots are parted by around 2-3 months. "You'll be hearing some good news from the UK very soon... It would be a 90-95% effective vaccine if you just keep a two-to-three months' gap between dose 1 and dose 2. They will make that public with documentation."
Covishield, Poonawalla added, is highly effective vaccine against novel coronavirus. The vaccine is being touted as one of the most promising vaccines for India where cost and logistics play a big roll.
Covaxin
Covaxin has been developed by Indian biotechnology company Bharat Biotech and clinical research body Indian Council of Medical Research (ICMR). The NDTV report calls Covaxin an inactivated vaccine -- one of the oldest methods for vaccinating people – which means that whole, inactivated viruses are injected in the body to trigger an immune response. These whole batches of coronavirus must be grown, "killed" using a chemical or heat and then made into a vaccine, making it a longer process, the report says.
According to reports, the recipient of the vaccine will also be handed fact sheet and an adverse effect reporting form where they would have to note down symptoms suffered within the first seven days. The consent form state that the vaccine has demonstrated the ability to produce antibody against coronavirus in phase 1 and phase 2 clinical trials. “However, the clinical efficacy of Covaxin is yet to be established and it is being studies in phase 3 clinical trial,” the report said.
Source and more reading here: Covishield vs Covaxin: What We Know About Efficacy of the Two Coronavirus Vaccines in India
https://www.news18.com/news/india/corona...97032.html
Source and more reading here: Covaxin or Covishield? A dilemma for India's healthcare workers
https://www.straitstimes.com/asia/south-...re-workers
Source and more reading here: 5 killed in blaze at Indian producer of COVID-19 vaccine
https://apnews.com/article/pune-india-co...dd257fad6b
Solidarity trials are underway in every corner of the globe. The COVAX facility of the WHO is running and has paved way for 3rd-world countries to have at the very least "free vaccines" for their use. I hope (and pray) that the pharmaceutical firms will not get greedy much less be complacent to stop development because the one thing that we do not want is a "mutation" that is more infectious and deadly. At this time, all are testing (or some already started) their vaccine's effectivity versus the mutated UK variant and the South African variant. The boast and flak will definitely come as the manufacturer's try to outdo each other again (based on this mutations). It's a wait and see game (still)...
Posts: 304
Threads: 30
Thanks Received: 313 in 211 posts
Thanks Given: 180
Joined: 26 October 18
I was checking the WHO earlier and they not have info on pregnant women..Also there are a lot of negative news going around of the vaccine's ineffectivity. This contribute to some that are confused and so are indignant to be injected. What do you prefer? In the news it seems that Sputnik 5 has the best effectivity. Anyway in our region the government will be the one to select for us. If we do not want what they give they said we should buy our own. 
Posts: 34
Threads: 6
Thanks Received: 28 in 23 posts
Thanks Given: 9
Joined: 04 April 19
Omicron and the BA.2 Subvariant: A Guide to What We Know
https://www.yalemedicine.org/news/5-thin...ow-omicron
|
Users browsing this thread: 1 Guest(s)
|
|
Welcome
|
You have to register before you can post on our site.
|
|
Recent Posts
|
|
AdGuard VPN for Windows 2.9
|
|
AdGuard VPN for Wi...harlan4096 — 10:58 |
|
Rufus 4.13
|
|
Rufus 4.13
Fi...harlan4096 — 10:57 |
|
QOwnNotes
|
| 26.2.6
Improved ...Kool — 10:30 |
|
Internet Download Manager 6.32 Build 9
|
|
Internet Download ...Kool — 09:14 |
|
K-Lite Codec Pack 19.5.0 / 19.5.1 Update
|
|
Changes in 19.5.0:...harlan4096 — 08:41 |
|
Birthdays
|
|
Today's Birthdays
|
 (51)Ronaldduh
(51)Ronaldduh
|
 (39)legalgauch
(39)legalgauch
|
|
Upcoming Birthdays
|
 (38)showercurtains
(38)showercurtains
|
 (49)PeterWhink
(49)PeterWhink
|
 (46)dimaWeami
(46)dimaWeami
|
 (38)Michaelaburi
(38)Michaelaburi
|
 (46)dpascoal
(46)dpascoal
|
 (44)Baihu
(44)Baihu
|
|
Online Staff
|
| There are no staff members currently online. |

|
|

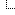



![[-]](https://www.geeks.fyi/images/collapse.png)
![[Image: 2021-02-09-004548.png]](https://i.postimg.cc/MGK8Q4XN/2021-02-09-004548.png)
![[Image: vaccine-2.png]](https://i.postimg.cc/mDz5hCZM/vaccine-2.png)
![[Image: vaccine-1.png]](https://i.postimg.cc/3wbqKnhY/vaccine-1.png)